[ad_1]
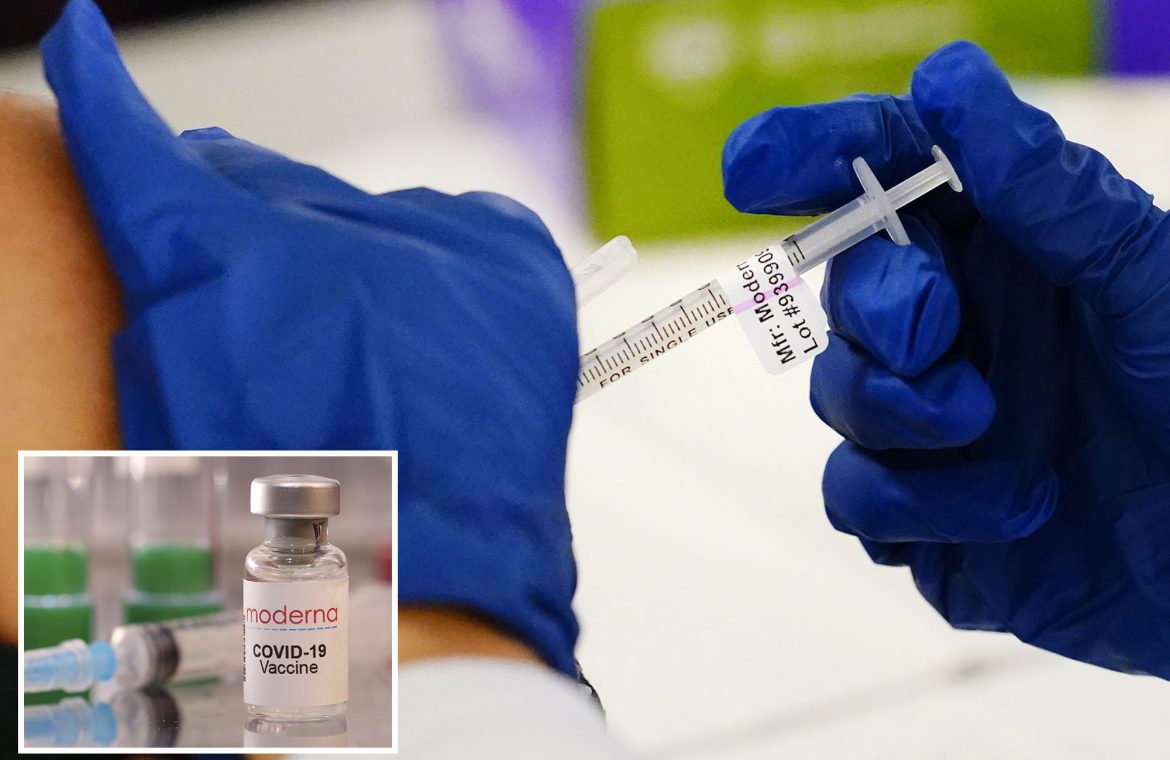
Moderna’s COVID-19 vaccine works effectively in babies, toddlers and preschoolers, the company announced Wednesday.
Based on results from its clinical trial, the drugmaker said it now plans to ask regulators in the coming weeks to authorize two small-dose shots for children younger than 6 years.
If approved by the Food and Drug Administration, it could mean a chance to finally start vaccinating young children by summer.
“Given the need for a vaccine against COVID-19 in infants and young children we are working with the US FDA and regulators globally to submit these data as soon as possible,” Moderna CEO Stéphane Bancel said in a statement.
The nation’s 18 million children aged under 5 are the only age group not yet eligible to receive a COVID vaccine.
If approved by the FDA, young children could be vaccinated beginning in the summer.AFP via Getty Images
The news comes based on the results of the drug-maker’s clinical trial.AP
Pfizer currently offers kid-sized doses for school-age children and full-strength shots for those aged 12 and older.
Moderna said its clinical trial found a quarter of the dose it uses for adults worked well for youngsters under age 6.
Early data from the trial showed after two shots, those under 6 developed virus-fighting antibody levels just as strong as young adults getting regular-strength shots, Moderna said.
The nation’s children aged under 5 are the only group not eligible to receive a COVID vaccine.AFP via Getty Images
Moderna stated its COVID-19 vaccine was safe and effective for kids 6 to 11 back in Oct. 2021.AP
The main side effects were mild fevers like those associated with other commonly used pediatric vaccines, the company added.
Moderna said it is also seeking to have larger-dose shots cleared for older children and teens in the US, based on additional evidence from studies.
The company said its original adult dose — two 100-microgram shots — is now safe and effective in 12- to 17-year-olds. For elementary-age children, Moderna said half the adult dose is safe.
Moderna said it is also seeking to have larger-dose shots cleared for older children and teens.REUTERS
The FDA, however, has so far not given clearance for Moderna’s application to increase the dose for teens over covers about a rare side effect
FDA never ruled on Moderna’s application for teen shots because of concern about a very rare, heart inflammation side effect.
[ad_2]
Source link